Rheumatologists Dr. Jill P. Buyon and Dr. Peter M. Izmirly have teamed up to study the risks and benefits of hydroxychloroquine withdrawal in older patients with stable lupus.
Photo: NYU Langone Staff
A new line of research led by scientists at NYU Langone Health is investigating whether it is safe to withdraw hydroxychloroquine (prescribed as Plaquenil®) therapy in older lupus patients with stable or quiescent disease, since the drug is associated with ocular toxicity over time.
A Growing Focus on Long-Term Safety Concerns
Hydroxychloroquine (HCQ) is considered the mainstay of systemic lupus erythematosus (SLE) treatments. Studies have suggested that HCQ can prevent or forestall organ damage such as kidney involvement, possibly have beneficial cardiovascular effects, and may even lower mortality rates. Among older SLE patients, however, accumulating evidence has fueled concern over the risk of ocular toxicity that can lead to blindness if not addressed. A drug withdrawal study is one of the best ways to evaluate how beneficial a drug really is and whether its continued use is warranted, says Jill P. Buyon, MD, the Sir Deryck and Lady Va Maughan Professor of Rheumatology and director of the Division of Rheumatology and Lupus Center at NYU Langone.
“There are virtually no studies that have looked at this population of older lupus patients,” Dr. Buyon says. “Knowledge is power, and as we achieve our goal of having patients live longer, we must now consider the potential toxicities of medications in aging populations.”
Research on other immunosuppressive drugs, like those for rheumatoid arthritis, suggests that toxicities can indeed build up over time.
“Knowledge is power, and as we achieve our goal of having patients live longer, we must now consider the potential toxicities of medications in aging populations.”—Jill P. Buyon, MD, Director of the Division of Rheumatology
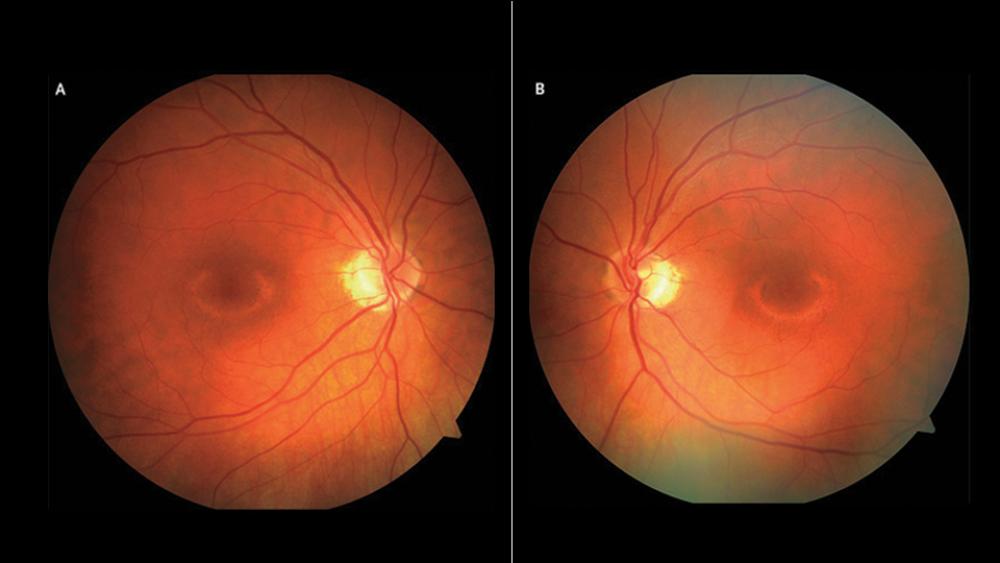
“Although traditionally thought to be a relatively safe medication, HCQ has been shown to have an ophthalmologic toxicity,” says Peter M. Izmirly, MD, associate professor of medicine. At first, doctors thought the toxicity was relatively rare and of concern mainly in patients taking the medication on a long-term basis, he says. But more sensitive testing has suggested that the risk is much higher than previously thought. Evidence of increased cardiomyopathy risk has begun to accrue as well, though the rate remains unknown for patients on HCQ therapy.
Retrospective Study Suggests HCQ Withdrawal Is Safe in Older Patients
After encountering both eye and heart toxicities in patients, Dr. Izmirly says, “I began to wonder if maybe the benefits of the drug that we see in younger, more active lupus patients aren’t there as much in older quiescent patients.” Multiple discussions with Dr. Buyon ensued, and the researchers teamed up to study the risks and benefits of HCQ withdrawal in older patients with stable disease.
In an initial retrospective study, the researchers assessed the outcomes of 27 lupus patients who had been on HCQ for at least 5 years before discontinuing it for a variety of reasons. The patients were at least 55 years old at the point of withdrawal, and nearly half stopped taking the drug after developing maculopathy.
Compared with a control group of 39 patients who remained on HCQ and were matched for age, gender, race, and ethnicity, the researchers showed that stopping the drug had no effect on the risk of moderate or severe lupus flares within 1 year of withdrawal. “These data suggested that it was relatively safe to withdraw the medication in this stable older population,” Dr. Izmirly says.
Large Prospective Study Planned to Further Assess HCQ Safety
With the aid of a National Institutes of Health (NIH) planning grant, the researchers hope to expand their safety assessment through a prospective, multicenter trial that will enroll about 330 patients who are at least 60 years old. “We will take older non-active lupus patients and randomize half to stop the medication and the other half to continue the medication,” Dr. Izmirly says. “We will follow them for a year and see if this older population has any increased risk of flares.”
Both Dr. Izmirly and Dr. Buyon caution that they wouldn’t stop prescribing HCQ in young patients with active lupus unless forced to do so due to significant side effects. “But in older patients who’ve been on it for a long time, maybe the risk–benefit ratio is tilting more toward the risk than we previously thought,” Dr. Izmirly says. By carefully following patients after HCQ withdrawal, the team can begin to address that question.
“Accordingly, we’re addressing a very timely and critical consideration that is increasingly facing clinicians caring for older adults with SLE: Can HCQ be safely withdrawn?” Dr. Buyon says. “This question has even more relevance given the minimal attention that has been focused on managing SLE in the aging patient population. Just as physicians are seeking new therapies in an ever-expanding landscape of biologics, we see the need to adjust medications and address withdrawal as critical components of patient care.”
Disclosure: Peter M. Izmirly, MD, is on the scientific advisory board for GlaxoSmithKline.